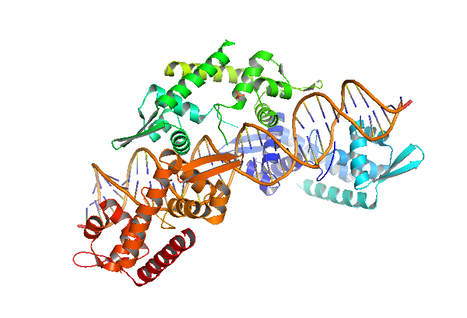
The precise distribution of newly replicated genomes to progeny cells is crucial for stable transmission of genetic information. Bacteria and Archaea employ dedicated DNA segregation factors that drive and deliver low copy number plasmids and chromosomes to specific subcellular locations. We are interested in the mechanisms underpinning the segregation of chromosome and low copy number plasmids in procaryotes. Different research strands are currently ongoing in our lab.
Segregation of a multidrug resistance plasmid
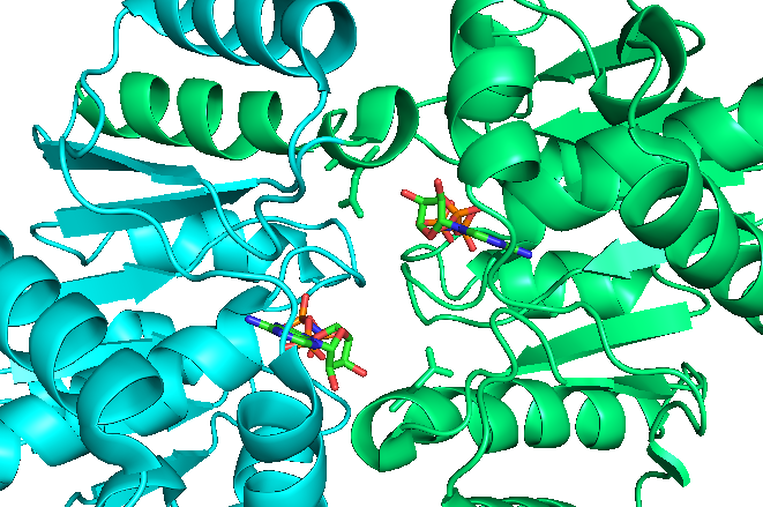
Structure of ParF dimer
Bacterial antibiotic resistance is a growing burden on human health worldwide. The emergence of multidrug-resistant 'superbugs' among bacterial populations results from mutations within the chromosome or from the horizontal transfer of resistance genes present on mobile genetic elements such as plasmids, bacteriophages, transposons and pathogenicity islands. The mobility of these genetic entities is key for intra-species and worringly inter-species dissemination of multidrug resistance.
One of the model systems under investigation is the multidrug resistance plasmid TP228 that replicates at low copy number in Escherichia coli. This mobile genetic element specifies resistance to a range of antibiotics, including tetracycline, streptomycin and sulphonamides. The plasmid harbours a partition cassette encoding two proteins that are crucial for plasmid inheritance: ParF, a ParA Walker-type ATPase, and ParG, a site-specific DNA-binding protein. We employ multidisciplinary approaches, spanning from genetics to biochemistry, from super resolution microscopy to biophysics, to investigate the role and interactions of these proteins in mediating plasmid segregation.
One of the model systems under investigation is the multidrug resistance plasmid TP228 that replicates at low copy number in Escherichia coli. This mobile genetic element specifies resistance to a range of antibiotics, including tetracycline, streptomycin and sulphonamides. The plasmid harbours a partition cassette encoding two proteins that are crucial for plasmid inheritance: ParF, a ParA Walker-type ATPase, and ParG, a site-specific DNA-binding protein. We employ multidisciplinary approaches, spanning from genetics to biochemistry, from super resolution microscopy to biophysics, to investigate the role and interactions of these proteins in mediating plasmid segregation.
Dynamics of a plasmid encoding virulence factors
Plasmid pB171 is a 70 kb, low copy number plasmid harboured by an enteropathogenic Escherichia coli strain, designated as B171. Enteropathogenic E. coli is a serious threat to human health worldwide, with more devastating consequences in countries of the developing world. It causes diarrheal diseases by colonizing the small intestine through attachment of bacteria to epithelial cells. The localized adherence of bacteria clusters causes the formation of pedestal-like structures in the epithelial cells and determines the loss of nearby microvilli, also known as effacing phenotype. The ability to adhere to epithelial cells is mediated by adherence factors encoded by plasmid pB171. A study has demonstrated that a plasmid-cured enteropathogenic E. coli strain was significantly less pathogenic than the parental strain. Therefore, investigating the stability and segregation mechanisms of this plasmid will pave the way to devising possible strategies to target the partition apparatus and thus cure the plasmid from pathogenic strains.
The partition cassette of pB171 consists of two genes, parA, which encodes a Walker-type ATPase, and parB, which encodes a DNA-binding protein recognizing a set of direct repeats upstream of parA. Genetic and structural investigations are currently ongoing in the lab to define properties and mechanism of action of ParA and partner protein ParB.
The partition cassette of pB171 consists of two genes, parA, which encodes a Walker-type ATPase, and parB, which encodes a DNA-binding protein recognizing a set of direct repeats upstream of parA. Genetic and structural investigations are currently ongoing in the lab to define properties and mechanism of action of ParA and partner protein ParB.
Chromosome segregation in Archaea
Archaea are the third domain of life: these organisms are widely disseminated in the most disparate environmental niches and present unique molecular adaptations to life pushed to extremes. Thermophilic archaea are super microbes thriving at 80 degrees C and higher temperatures in hot springs, volcanoes, and deep sea vents. We work on the thermophilic archaeon Sulfolobus solfataricus, that was originally isolated from the solfatara fields located in Campi Flegrei ('Burning Fields') in Naples, Italy.
Chromosome segregation is a fundamental biological process in all organisms. It requires the concerted action of dedicated proteins and its coordination with cellular events, such as DNA replication and cell division, is crucial to maintain euploidy. The molecular mechanisms promoting chromosome separation in eukaryotes are well characterized. Considerable progress has been made to decipher this process in bacteria in the last three decades. In contrast, the molecular mechanisms and factors underpinning chromosome segregation in archaea remain a black box awaiting investigation.
We have shown that Sulfolobus solfataricus harbours a hybrid segregation machine consisting of two interacting proteins, SegA and SegB, that play a key role in chromosome segregation in this organism. SegA is an ortholog of bacterial Walker-type ParA proteins, whereas SegB is an archaea-specific factor that works as a DNA anchor by binding to palindromic motifs. The picture emerging from our findings indicates that the SegAB complex fulfills a crucial function in chromosome segregation and is the prototype of a DNA partition system widespread across archaea.
Chromosome segregation is a fundamental biological process in all organisms. It requires the concerted action of dedicated proteins and its coordination with cellular events, such as DNA replication and cell division, is crucial to maintain euploidy. The molecular mechanisms promoting chromosome separation in eukaryotes are well characterized. Considerable progress has been made to decipher this process in bacteria in the last three decades. In contrast, the molecular mechanisms and factors underpinning chromosome segregation in archaea remain a black box awaiting investigation.
We have shown that Sulfolobus solfataricus harbours a hybrid segregation machine consisting of two interacting proteins, SegA and SegB, that play a key role in chromosome segregation in this organism. SegA is an ortholog of bacterial Walker-type ParA proteins, whereas SegB is an archaea-specific factor that works as a DNA anchor by binding to palindromic motifs. The picture emerging from our findings indicates that the SegAB complex fulfills a crucial function in chromosome segregation and is the prototype of a DNA partition system widespread across archaea.
A DNA segregation model system from Archaea with bacterial and eukaryotic connections
Sulfolobus NOB8H2 is a strain isolated by the archaea pioneer Wolfram Zillig from acidic hot springs at Noboribetsu in the island of Hokkaido, Japan. This strain harbours a 41 kbp conjugative plamid, pNOB8, that was sequenced by Qunxin She and colleagues. The plasmid harbours 50 genes of which some encode proteins related to plasmid functions like conjugation and two encode proteins resembling bacterial ParA and ParB. We identified a small gene upstream of parA and overlapping with its 5' end to which we gave the name of aspA.
We have investigated this atypical, three-component DNA segregation system and structural studies have disclosed bacterial and eukaryotic linkages. This genome partitioning machine consists of a complex including a Walker-type ParA, a chimeric ParB adaptor and a unique centromere-binding factor, AspA. The AspA protein spreads from its binding site on the DNA, generating a helical docking platform onto which the N-terminal domain of ParB assembles fitting in a lock-and-key fashion into the AspA-DNA structure. Surprisingly, the ParB C-terminus, which binds non-specific DNA, shows structural similarity to the CenpA histone variant, which demarcates centromeres and is vital for kinetochore assembly in eukaryotic cells. ParA is recruited onto the complex via interaction with ParB. This multi-protein structure merges bacterial and eukaryotic features, suggesting the possible conservation of DNA segregation principles across the domains of life.
We have investigated this atypical, three-component DNA segregation system and structural studies have disclosed bacterial and eukaryotic linkages. This genome partitioning machine consists of a complex including a Walker-type ParA, a chimeric ParB adaptor and a unique centromere-binding factor, AspA. The AspA protein spreads from its binding site on the DNA, generating a helical docking platform onto which the N-terminal domain of ParB assembles fitting in a lock-and-key fashion into the AspA-DNA structure. Surprisingly, the ParB C-terminus, which binds non-specific DNA, shows structural similarity to the CenpA histone variant, which demarcates centromeres and is vital for kinetochore assembly in eukaryotic cells. ParA is recruited onto the complex via interaction with ParB. This multi-protein structure merges bacterial and eukaryotic features, suggesting the possible conservation of DNA segregation principles across the domains of life.